The study aimed to evaluate the effectiveness of a novel probiotic, Bacillus subtilis HU58, in patients with Antibiotic-Associated Diarrhea (AAD) through an open-labeled placebo-controlled trial with 60 participants. The patients received either Probiotic Bacillus subtilis HU58 or a placebo for 7 days and were followed up to the 15th day. Stool consistency, as measured by the Bristol stool chart, showed a significant improvement in the probiotic-treated group compared to the placebo group. The probiotic group demonstrated a reduction in stool frequency and improved stool consistency, indicating that Bacillus subtilis HU58 may be a promising treatment for AAD.
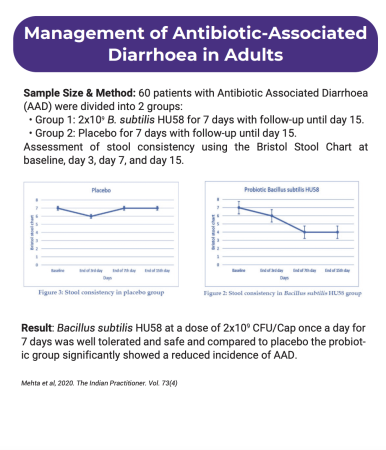
Antibiotic-associated diarrhea (AAD) is a common healthcare issue, and the probiotic Bacillus subtilis HU58 has demonstrated effective control for AAD. In a study, AAD patients who received Probiotic Bacillus subtilis HU58 at a dose of 2 X 109 CFU/Cap once a day for 7 days experienced significant improvement in stool consistency compared to the placebo group. The probiotic was well-tolerated and safe in all patients. However, further evaluation in a larger sample size through a placebo/active-controlled double-blind randomized multi-centric trial is needed to determine its full therapeutic efficacy.














