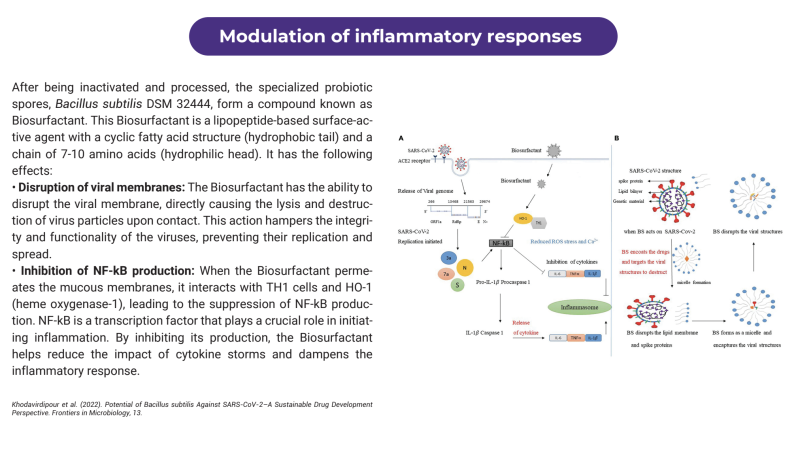
The postbiotic of Bacillus subtilis DSM 32444 can be used as a nasal spray to effectively prevent respiratory viruses in humans and animals
Probiotic strain Bacillus subtilis DSM 32444
The probiotic strain Bacillus subtilis 32444 was researched and developed using specialized technology:
A collaborative research project by Sporegen Biotechnology Company and Destiny Pharma Pharmaceutical Company; Reviewed, funded, and strictly controlled by UKRI organization under the UK Government.
Rigorous safety assessment tests are carried out at the UK’s leading prestigious Lab – Charles River Laboratories.
Efficacy evaluation studies were also conducted at the University of Liverpool (UK), and Royal Holloway University (UK).
Bacillus subtilis DSM 32444K achieved absolute safety certification (GRAS) approved by the US Food & Drug Administration (FDA).

Respiratory health